Abstract
Background:
To help optimize decision-making for acute myeloid leukemia (AML) treatment in the clinic, risk models comprising different variables were developed. A Treatment-Related Mortality (TRM) model was developed to predict 28-day mortality. The TRM model includes age, performance status, platelet count, albumin, secondary AML, peripheral white blood cell count, peripheral blood blast percentage, and creatinine (Walter et al, 2011 Nov 20; 29(33): 4417-4424). More recently, an AML-specific composite model (AML-CM) introduced the combination of cytogenetic/molecular risks per European Leukemia Network (ELN) classification with burdens of increasing age and comorbidities. The AML-CM can predict both early and late mortality after diagnosis of AML (Sorror et al, 2017; 3(12):1675-1682). All factors within the TRM model were tested during the development of the AML-CM, except for the impacts of Karnofsky Performance Status (KPS), and presence of secondary AML. To this end, we explored the possibility of augmenting the AML-CM by testing the prognostic value added by including KPS and presence of secondary AML.
Methods:
The study comprises two cohorts from two different institutions: 311 patients (pts) to be used for development of new scores and 187 pts to be used for independent validation. Both cohorts were seen for initial therapy for AML between 2008 and 2017. We collected patients' demographics and comorbidity information through patient charts. We defined secondary AML as an occurrence of AML after a previous malignancy or after hematological abnormalities for at least one month. Afterwards, Cox regression models were designed to test whether KPS and secondary AML have independent prognostic impacts that could be added to the AML-CM. Prediction of eight-week and one-year mortality were compared between the original versus augmented AML-CM using c-statistics and area under the curve (AUC) of the receiver-operator characteristic curve (ROC) in the independent validation cohort from the different institution.
Results:
In a multivariate model, after adjusting for AML-CM variables, both secondary AML (hazard ratio [HR] =2.19, p=0.0001) and worse KPS percentages (KPS 80-75%: HR=1.53, p=0.06; KPS ≤70%: HR=2.60, p=0.0001) were significantly associated with one-year mortality. Using the same equation used in the development of the AML-CM, we assigned 1 point to KPS 80-75%, 2 points to KPS ≤70% and 2 points to secondary AML. These points were added to those of age, comorbidities and ELN risks in order to calculate the augmented AML-CM scores.
In the validation set, the augmented AML-CM had a c-statistic estimate of 0.71 vs. 0.69 for the original AML-CM. The true AUC for prediction of 1-year mortality was 0.75 vs. 0.72 while the AUC for prediction of 8-week mortality was 0.83 vs. 0.79 for the augmented AML-CM and original AML-CM, respectively.
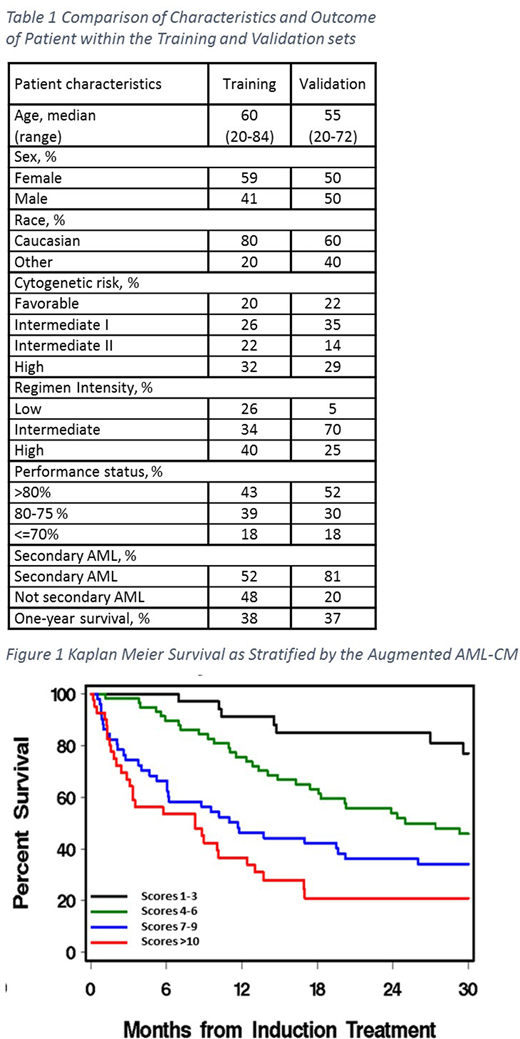
Figure 1 shows the discrimination of four risk groups per the new augmented AML-CM scores. Patients with scores of 1-3, 4-6, 7-9, and ≥10 had 1-year survival rates of 91%, 76%, 46% and 37%, respectively.
Conclusions:
In this study we found that worse KPS percentages add prognostic value to those of age and comorbidities, while secondary AML adds prognostic value to that of ELN cytogenetic/molecular risks for prediction of early (8-week) and late (1-year) mortality after diagnosis of AML. This suggests that KPS provides prognostic information above what is provided by age and comorbidities alone. Similarly, the classification of a patient's AML as secondary AML provides prognostic information beyond what is understood about the patient's disease risk as indicated by the ELN cytogenetic/molecular risk category.
Combining these five factors: age, comorbidity burden, performance status, disease risk, and presence of secondary AML results in an augmented AML-CM that can be used in the clinic for improved risk-assessment before treatment decisions.
Becker:GlycoMimetics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal